|
This article is cited in 16 scientific papers (total in 16 papers)
Synthesis and biological activity of aza and deaza analogues of purine nucleosides
E. S. Matyugina, S. N. Kochetkov, A. L. Khandazhinskaya
Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, Moscow
Abstract:
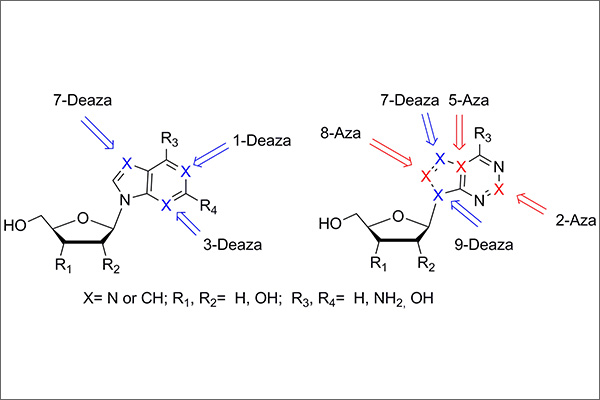
Analogues and derivatives of nucleic acid components have been used as key drugs in different areas of medicine over the past decades. The replacement of one or more nitrogen atoms of the heterocyclic base with a CH group affords deazapurine nucleoside analogues, and the replacement of the methine group with a nitrogen atom gives azapurine derivatives. A combination of aza and deaza moieties in the purine base leads to aza(deaza)-modified bases. Some nucleoside analogues were found to exhibit pronounced anticancer and antiviral activity. The synthesis and evaluation of biological activity of aza- and deazapurine nucleoside analogues have attracted interest from researchers four decades ago. This review describes and integrates the studies concerning certain aspects of the synthesis and(or) activity of various representatives of this class of compounds. The structure–biological activity relationships are analyzed. The successful approaches to the design of aza- and deazapurine nucleoside analogues are considered. A comparison is given for the methods of chemical and enzymatic synthesis of these compounds.
The bibliography includes 161 references.
Keywords:
aza/deaza purine nucleosides, heterocyclic base, carbohydrate fragment, biological activity.
Received: 04.12.2020
Citation:
E. S. Matyugina, S. N. Kochetkov, A. L. Khandazhinskaya, “Synthesis and biological activity of aza and deaza analogues of purine nucleosides”, Usp. Khim., 90:11 (2021), 1454–1491; Russian Chem. Reviews, 90:11 (2021), 1454–1491
Linking options:
https://www.mathnet.ru/eng/rcr4369https://doi.org/10.1070/RCR5013 https://www.mathnet.ru/eng/rcr/v90/i11/p1454
|

| Statistics & downloads: |
| Abstract page: | 103 |
|






 Contact us:
Contact us: Terms of Use
Terms of Use
 Registration to the website
Registration to the website Logotypes
Logotypes







 Citation in format
Citation in format 
