|
This article is cited in 17 scientific papers (total in 17 papers)
New method for the synthesis of heterospin metal complexes with nitroxides
V. I. Ovcharenkoab, O. V. Kuznetsovaa
a International Tomography Center of the Siberian Branch of the Russian Academy of Sciences, Novosibirsk
b N.D. Zelinskii Institute of Organic Chemistry, Russian Academy of Sciences
Abstract:
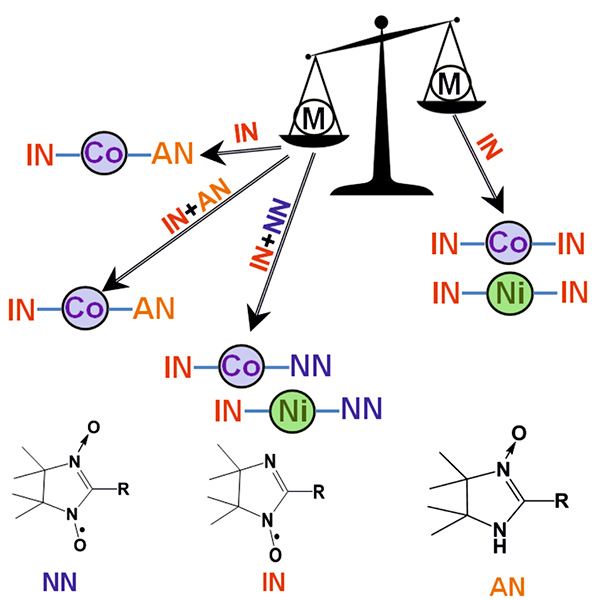
A new approach was developed to synthesize transition metal complexes with nitroxides based on the simultaneous involvement of nitronyl nitroxide and imino nitroxide in the reaction with metal. It was shown that the reaction of a metal with nitronyl nitroxide can afford a metal complex containing two different radicals in the metal coordination sphere in the case when imino nitroxide is generated in the reaction medium via a redox process. The reaction of a metal with imino nitroxide also can give mixed-ligand complexes, in which the metal is coordinated by both the starting imino nitroxide and its reduction product—the corresponding amidine oxide. This compound can be prepared by an independent synthesis using the reaction of metal with sterically hindered amidine oxide, resulting in the formation of mixed-ligand coordination compounds through the coordination of both the starting amidine oxide and its oxidation product—imino nitroxide. In the latter case, the following conditions have to be met: the reaction should be performed in the presence of oxygen and transition metal, which can easily change its oxidation state under ambient conditions ($\mathrm{Co}^{\mathrm{II}}$, $\mathrm{Mn}^{\mathrm{II}}, \mathrm{Fe}^{\mathrm{II}})$. To synthesize mixed-ligand complexes with transition metals, which are not prone to change the oxidation state under ambient conditions ($\mathrm{Ni}^{\mathrm{II}}$, $\mathrm{Zn}^{\mathrm{II}})$, a specially prepared mixture of nitronyl nitroxide and imino nitroxide should be added to the reaction mixture. It is worth noting that the reaction can be performed using nitronyl nitroxide and imino nitroxide belonging to different series, which significantly extends the scope of the method.
The bibliography includes 156 references.
Keywords:
Transition metal complex, Nitroxides, Redox-active ligands, New synthetic approach.
Received: 20.07.2020
Citation:
V. I. Ovcharenko, O. V. Kuznetsova, “New method for the synthesis of heterospin metal complexes with nitroxides”, Usp. Khim., 89:11 (2020), 1261–1273; Russian Chem. Reviews, 89:11 (2020), 1261–1273
Linking options:
https://www.mathnet.ru/eng/rcr4318https://doi.org/10.1070/RCR4981 https://www.mathnet.ru/eng/rcr/v89/i11/p1261
|


| Statistics & downloads: |
| Abstract page: | 83 |
|






 Contact us:
Contact us: Terms of Use
Terms of Use
 Registration to the website
Registration to the website Logotypes
Logotypes








 Citation in format
Citation in format 
