|
This article is cited in 13 scientific papers (total in 13 papers)
A. E. Favorskii's scientific legacy in modern organic chemistry: prototropic acetyleneallene isomerization and the acetylene zipper reaction
N. A. Danilkina, A. A. Vasileva, I. A. Balova
Saint Petersburg State University
Abstract:
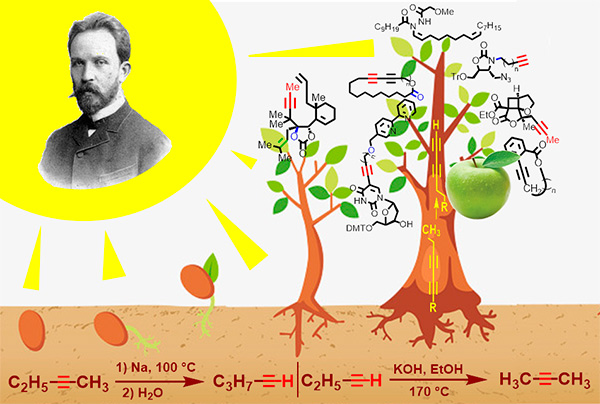
Alexei Evgrafovich Favorskii was an outstanding organic chemist who left a great scientific legacy as a result of long time and fruitful work. Most of the theoretically and practically important discoveries of A.E.Favorskii were made in the chemistry of acetylene and its derivatives. Nowadays, the reactions discovered by him, which include acetylene–allene isomerization, the Favorskii and retro-Favorskii reactions, the Favorskii rearrangement and the vinylation reaction, are widely used in industry and in laboratory synthesis. This review summarizes the main scientific achievements of A.E.Favorskii, as well as their development in modern organic chemistry. Much consideration is given to acetylene–allene isomerization as a convenient method for the synthesis of methyl-substituted acetylenes and to the acetylene zipper reaction as a synthetic tool for obtaining terminal acetylenes. The review presents examples of the application of these reactions in modern organic synthesis of complex molecules, including natural compounds and their analogues.
The bibliography includes 266 references.
Keywords:
acetylene-allene isomerization, metylacetylenes, "acetylene zipper", terminal acetylens, diacetylenes, Favorskii reaction, propargyl alcohols, retro-Favorskii reaction, Favorskii rearrangement.
Received: 30.05.2019
Citation:
N. A. Danilkina, A. A. Vasileva, I. A. Balova, “A. E. Favorskii's scientific legacy in modern organic chemistry: prototropic acetyleneallene isomerization and the acetylene zipper reaction”, Usp. Khim., 89:1 (2020), 125–171; Russian Chem. Reviews, 89:1 (2020), 125–171
Linking options:
https://www.mathnet.ru/eng/rcr4279https://doi.org/10.1070/RCR4902 https://www.mathnet.ru/eng/rcr/v89/i1/p125
|


| Statistics & downloads: |
| Abstract page: | 215 |
|






 Contact us:
Contact us: Terms of Use
Terms of Use
 Registration to the website
Registration to the website Logotypes
Logotypes







 Citation in format
Citation in format 
