|
This article is cited in 15 scientific papers (total in 15 papers)
Acetylene–azide click macrocyclization of peptides
E. A. Zakharova, O. I. Shmatova, V. G. Nenajdenko
Lomonosov Moscow State University, Faculty of Chemistry
Abstract:
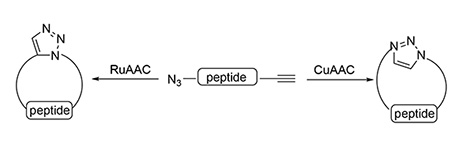
This review is focused on the synthesis of peptide macrocycles based on the intramolecular click reaction — [2+3]-cycloaddition of alkynes and azides giving a 1,2,3-triazole moiety. The importance of this class of macrocyclic peptides and the general approach to the synthesis of these compounds are discussed. The review summarizes published data on these reactions proceeding in the presence of catalysts based on copper(I) and ruthenium(II) salts and thermal cyclization reactions that allow the synthesis of 1,4- and 1,5-disubstituted 1,2,3-triazoles. Special attention is given to the Ugi/click strategy for the synthesis of macrocyclic peptides. This approach is shown to be efficient for the preparation of various macrocycles.
The bibliography includes 111 references.
Received: 25.01.2018
Citation:
E. A. Zakharova, O. I. Shmatova, V. G. Nenajdenko, “Acetylene–azide click macrocyclization of peptides”, Usp. Khim., 87:7 (2018), 619–635; Russian Chem. Reviews, 87:7 (2018), 619–635
Linking options:
https://www.mathnet.ru/eng/rcr4215https://doi.org/10.1070/RCR4788 https://www.mathnet.ru/eng/rcr/v87/i7/p619
|


| Statistics & downloads: |
| Abstract page: | 157 |
|





 Contact us:
Contact us: Terms of Use
Terms of Use
 Registration to the website
Registration to the website Logotypes
Logotypes







 Citation in format
Citation in format 
